After last week’s post about pus in semen, I thought that it might be helpful to describe another important sperm test, one that shows whether or not they’re alive.
Good sperm are alive. Not only do they swim, they live and breathe just as all living cells do. Some are dead, meaning that the ship is no longer sailing, and its motor and crew are gone.
One way of figuring out whether or not sperm are alive is to dip them in colored dye. A dead sperm can’t push the dye out of its body, but a live sperm can. In the picture, the sperm are stained with a pink dye called Eosin. The dead sperm are the ones that become pink, and the live sperm are the ones that push the dye outside and stay clear (a light bluish green in the photograph.)
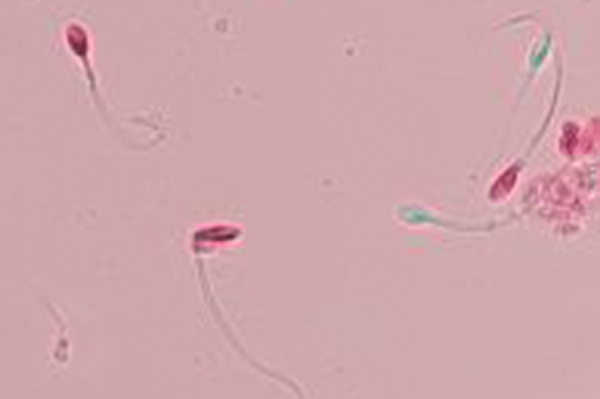
The result of the test is typically described in the percentage of sperm that are alive, and of course, the more living sperm the better. But even when all of the sperm are dead, a condition called “necrospermia”, couples can still conceive using in-vitro fertilization with intra-cytoplasmic sperm injection.
That doctors can successfully use dead sperm with in-vitro fertilization illustrates a conundrum in reproductive medicine today. The ultimate barrier to fertilization is no longer the entire sperm’s health, but the quality of its DNA cargo. Unfortunately, we don’t yet have a way of knowing the condition of a single sperm’s DNA before inserting that sperm into an egg.
So if a man’s sperm don’t wiggle, the first thing to know is whether they’re alive and sluggish or whether they’re dead. A vital stain makes that distinction. But if they’re dead, a man shouldn’t despair. Dead sperm can still be used in intra-cytoplasmic sperm injection as long as their DNA is good. Unfortunately, the only way to know that right now is to inject the sperm and to see what happens.
Has it really been over three months since my last post? Between becoming one of the next Co-Editors in Chief of Fertility and Sterility, preparing for a review of our urology training program and finishing my latest book (Thank You, Chapter Authors!) I guess that I’ve let my blogging slip a bit. Fortunately, thanks to my Italian co-faculty’s discovery of the Saeco Vienna Plus espresso maker at Costco, I’m back at the keyboard.
I turned off the two-week limit for comments, and so far, that’s been a good idea. People are commenting on older posts (like How Clomid Works in Men) with good questions and thoughtful points. For new commenters, please read the FAQ. I can’t answer questions about specific patients. Those are best left to a live visit with a doctor with an interest in male reproductive medicine. One great resource is the American Society for Reproductive Medicine’s Society for Male Reproduction and Urology page and the ASRM’s find a doctor search page, (just click on the “Society for Male Reproduction and Urology (SMRU)” button in the “Find Member by Affiliated Society:” section.) Another excellent way to find a specialist who treats men with reproductive issues is to use the American Urological Association’s Society for the Study of Male Reproduction’s search engine.
This blog post was inspired by several patients who asked after I explained surgical sperm retrieval, if there was somewhere they could go for more information. I realized that I hadn’t written about such a common issue.
Just as a carpenter has many ways to make a cabinet, a surgeon can tackle a problem in a number of ways. And just as two cabinets may differ, different surgical problems demand different approaches. Such is the case in retrieving sperm from the testis.
Most of the time, taking sperm directly from the testis is necessary when a man has azoospermia, where no sperm is found in the ejaculate. Azoospermia takes two basic forms, obstructive and non-obstructive. As the name implies, obstructive azoospermia is due to a blockage in the tubes and structures that convey sperm from the testis to the outside world. In the best case, a surgeon can fix the errant anatomy, allowing a couple to conceive children without further ado. But because the tubes are so tiny, sometimes the tubes can’t be reconnected with surgery, and the alternative is to take sperm from the testis for it to be used in in-vitro fertilization.
The other form of azoospermia, non-obstructive, arises when the factory making sperm in the testis isn’t working quite right. Sometimes, the cells starting sperm are missing entirely, a condition known as “Sertoli cell only syndrome”. Occasionally, sperm may be rolling along their assembly line, a process that takes two to three months to complete, and stop mid-production. When that happens, it’s called “maturation arrest“. But frequently, sperm can be found in small amounts in the testis and can be retrieved using surgery.
 Because it isn’t mature, sperm from the testis can only be used with in-vitro fertilization and intra-cytoplasmic sperm injection.
Because it isn’t mature, sperm from the testis can only be used with in-vitro fertilization and intra-cytoplasmic sperm injection.
How can a surgeon remove sperm? He or she can take it from the testis itself, or the epididymis, the tiny coiled tube lying on the back of the testis where sperm mature. The surgeon can insert a needle into the testis or epididymis, he or she may make one or several small incisions into the testis or use microsurgery to retrieve sperm from either the testis or epididymis. In the case of obstructive azoospermia, it doesn’t seem to matter which technique is used. There’s plenty of sperm wherever it’s sought, and any method will do to retrieve it. When a man has obstructive azoospermia, I usually recommend taking a small piece from the testis, as the sperm may be frozen and is good for a number of in-vitro fertilization cycles so that the man doesn’t need to go through a procedure for every cycle, and can be there for his wife during her procedures.
 We’ve found that frozen sperm is just as good as fresh. in fact, the chances for fertilization are the same for fresh and frozen sperm, and the chance for pregnancy may even be a little better for frozen sperm than for fresh.
We’ve found that frozen sperm is just as good as fresh. in fact, the chances for fertilization are the same for fresh and frozen sperm, and the chance for pregnancy may even be a little better for frozen sperm than for fresh.
 Frozen sperm should literally last forever. It’s in liquid nitrogen, which is so cold that the building blocks making sperm don’t decay. Freezing sperm gives a couple time to plan when in-vitro fertilization is done.
Frozen sperm should literally last forever. It’s in liquid nitrogen, which is so cold that the building blocks making sperm don’t decay. Freezing sperm gives a couple time to plan when in-vitro fertilization is done.
When he has non-obstructive azoospermia, a man’s options are more limited. A surgeon can use the operating microscope to comb through the testis looking for areas that may contain sperm, a procedure known as “microsurgical testis sperm extraction“. Other techniques include making several small incisions in the testis or piercing the testis with a needle in a dozen or so different spots. When a man has non-obstructive azoospermia, I usually recommend microsurgical testis sperm extraction. More areas of the testis can be examined, and I can see the places that most likely contain sperm.
We’ve observed that prescribing a man with non-obstructive azoospermia clomiphene citrate for a few months before surgical retrieval seems to increase the chance to retrieve sperm. In many men, sperm appears in the ejaculate and surgery isn’t needed. If a couple has a few months, taking clomiphene before surgical sperm retrieval might be a good idea.
In short, a surgeon has many ways to retrieve sperm when necessary. The choice depends on the preference of the surgeon and the couple, and what’s going on inside the testis. I’ve listed the surgical techniques available, and my typical recommendations.
You may have heard the news that British biologist Robert Edwards won the Nobel prize in Medicine for his work developing in-vitro fertilization. Gynecologist Patrick Steptoe pioneered advances in retrieving and replacing a woman’s eggs without open surgery, and Edwards made breakthrough discoveries in keeping sperm and eggs alive outside of the body. Together, their work resulted in the first “test tube” baby born in 1978. She’s now a healthy adult with children of her own.
I clearly remember the breaking news of that first IVF baby. I was headed to college, and it read like science fiction. All sorts of horrors were imagined and published, alongside articles expressing the thrill of bringing children to childless couples. More than four million IVF babies have been born since that remarkable first, four million people who otherwise wouldn’t be here with us today.
Patrick Steptoe couldn’t share the Nobel prize with Robert Edwards, although he most certainly would have if he could. He died in 1988, ten years after his great success, and the prize isn’t awarded posthumously. Congratulations, Drs. Edwards and Steptoe. You not only brought us great science, you brought children into our homes.

